Summary Statement
This fact sheet reviews the hazards associated with skin exposure to chemicals and selecting the appropriate gloves.
INTRODUCTION
Hands and fingers are subject to an army of hazards on the farm. This fact sheet reviews the hazards associated with exposure to chemicals. Generally, exposure means contact through the skin or respiratory system, and ingestion. Research reveals that at least 80% of total body exposure to farm chemicals is to the hands.Once exposed, the person could be adversely like developing skin dermatitis or a burn from a corrosive chemical. Chemical can also be absorbed through the skin and into the body, causing a reaction that can lead to acute poisoning. General symptoms often associated with mild exposure to farm chemicals include headache, fatigue, dizziness, loss of appetite, stomach cramps, and diarrhea. Severe exposure to highly toxic compounds can lead to loss of coordination, seizures, and unconsciousness.
When working with agricultural and other chemicals, no single glove will protect your hands completely. Gloves made from polymers and other materials have their strengths and weaknesses in terms of preventing resistance and physical properties like resistance to tearing and abrasion. Since no protect-all polymer exists, selecting the right glove for the job is imperative to your safety.
SELECTIONThe selection of the proper chemical-resistant glove begins with an evaluation of the job application. Factors that influence this selection are:
- the type of chemicals to be handled (or used)
- frequency and duration of chemical contact
- nature of contact (total immersion or splash only)
- concentration of chemicals
- temperature of chemicals
- abrasion/resistance requirements
- puncture-, snag-, tear-, and cut-resistance requirements
- length to be protected (hand only, forearm, arm)
- dexterity requirements
- grip requirements (dry grip, wet grip, oily)
- cuff edge (safety cuff, knit wrist, or gauntlet)
- color requirements (to show contamination)
- thermal protection (for example, when handling anhydrous ammonia)
- size and comfort requirements
- price
The type of chemical being used is the key factor for choosing of what material the glove should be made. When possible use the farm chemical as the basis for the selection. With emulsifiable concentrates, volatile solvents (like toluene and xylene) and nonvolatile solvents (like alkylated napthalenes and petroleum oil) correct glove selection is critical. Some of the more common glove materials are:
- butyl - a synthetic rubber with good resistance to weathering and a wide variety of chemicals
- rubber - a highly flexible and conforming material made from a liquid tapped from rubber plants
- neoprene - a synthetic rubber having chemical and wear-resistance properties superior to those of natural rubber
- nitrile - a copolymer available in a wide range of acrylonitrile (propane nitrile) content; chemical resistance and stiffness increase with higher acrylonitrile content
- polyethylene - a fairly chemical-resistant material used as a freestanding film or a fabric coating
- polyvinyl alcohol - a water-soluble polymer that exhibits exceptional resistance to many organic solvents that rapidly permeate most rubbers
- polyvinyl chloride - a stiff polymer that is made softer and more suitable for protective clothing applications by the addition of plasticizers
- polyurethane - an abrasion-resistant rubber that is either coated into fabrics or formed into gloves or boots
- Server Shield - a registered trademark of North Hand Protection, it is highly chemical-resistant to many different class of chemicals
- Viton® - a registered trademark of DuPont, it is a highly chemical-resistant but expensive synthetic elastomer
For a given thickness, the type of polymer selected has the greatest influence on the level of chemical protection. For a given polymer an increase in thickness will result in a higher level of protection. A rule of thumb is that double the thickness will quadruple the breakthrough time.
The manufacturing process of glove making may result in slight variations in performance. The user is warned to exercise care and to check the glove regularly for breakthrough and diminished physical performance.
Physical performance may be a more critical factor in some cases than chemical resistance. If a job application involves handling heavy, rough, or sharp objects then the glove must have high resistance to abrasion, cuts, snags, etc. A hole in a glove can provide much greater chemical exposure potential than molecular permeation.
The thicker the glove material the greater the chemical resistance. But thick gloves can impair grip, dexterity, and safety. Consider sensitivity and the ability to grip as very important factors.
The proper glove design and fit contribute to comfort, productivity, and safety. Curved-finger glove design fits the natural hand contour for working comfort. Gloves that are too small bind and cause undue hand fatigue. However, gloves that are too large are uncomfortable, hard to work in and can be dangerous if they get caught in moving machinery.
Use the following steps in selecting the proper gloves when handling farm chemicals:
- Refer to manufacturer's Chemical Resistance Guide and Physical Performance Chart and select the glove type with the highest rating for the chemical and physical conditions. Also refer to the chemical label and the Material Safety Data Sheet (MSDS), which may recommend a specific glove type. One company writes "The information contained in these guides is advisory only. The purchaser must determine, by testing the product's suitability for use with the specific chemical."
- Select unsupported gloves for extra dexterity and sense of touch. An unlined glove is recommended to minimize exposure from contamination.
- Select a palm finish to provide the grip needed for the job-smooth, dipped, or embossed.
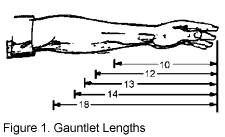
- Choose the glove length by the depth to which the arm will be immersed and by allowing for protection against chemical splash (see Figure 1).
- Select thin-gauge gloves for jobs demanding sensitive touch and high flexibility. If greater protection or durability is wanted, choose a heavy-duty style.
- Choose the glove size that will assure optimum wear, dexterity, working ease, comfort, and employee satisfaction.
Chemical resistance and physical performance charts are not included in this fact sheet because of the variance in manufacturers' recommendations. A good example is white gasoline. One manufacturer rated neoprene as an excellent glove for this, while another could not recommend it because of a high permeation rate. It is up to you to consult charts from specific glove manufacturers to make a safe decision.
The fact that little or no test data is available as yet for agricultural chemicals makes the selection from vendor literature for such application, at best, a difficult and uncertain task. Selecting a glove from a retailer or vendor catalog should be only the starting point. Further evaluation by the user is needed if chemical resistance is an issue.
USE AND CARE
Always inspect your gloves before using them. Of principal concern are cuts, tears and punctures. Discoloration or stiffness may indicate non-uniformities in the rubber or plastic or chemical attack resulting from previous use.
Visual inspection should be done every time you use the gloves to detect pinholes or other defects. One way to accomplish this is when they are still wet after having been washed, fill the glove with water and tightly roll the gauntlet toward the fingers and examine for leaks. Dispose of gloves that have been damaged or show signs of chemical degradation.
Proper handling of chemicals requires your wearing the gloves on the inside of your shirt sleeves. The exception is when you are working overhead and when your hands are in an upward position. In that case, put your shirt sleeves inside the gloves and turn up the cuff of the glove to catch any material that may run down your arm.
It is extremely important to avoid secondary exposure to the chemical after application. Before removing the gloves, thoroughly wash gloves with soap and water, or a detergent and water, and then rinse with a lot of running water. The gloves may now be removed. As the gloves dry in a decontaminated area, thoroughly wash your hands with soap and water. Make this a strict practice after every chemical application. Place dry gloves in a sealed plastic bag or other container, and store away from possible contamination.
REUSE QUESTIONGlove decontamination and reuse are controversial and unresolved issues. Often, surface contamination can be removed by scrubbing with soap and water; at other times, as in the case of emulsifiable concentrates, it may be practically impossible. The solvents in many emulsifable concentrates prompt this concern. Volatile solvents such as toluene and xylene, readily penetrate many polymers and the nonvolatile solvents, such as alkylated napthalenes and petroleum oil, are very difficult to remove from the glove material.
Once absorbed, some chemicals will continue to diffuse through the material toward the inside EVEN AFTER the surface has been DECONTAMINATED. For highly resistant chemical gloves, the amount reaching the inside may be insignificant. But for moderately performing materials, significant amounts of chemicals reach the inside. This may not occur during use, but while the glove is stored overnight. The next morning, when the applicator dons the glove, he may be putting his hand into direct contact with a hazardous chemical. In addition to the chemical resistance of the glove material, the amount of chemical that reaches the surface can be affected by the duration of exposure, duration of storage, the surface area exposed, and temperature.
The decision to reuse the gloves requires consideration of these factors as well as the toxicity of the chemical(s). In fact, unless extreme care is exercised to ensure decontamination, the reuse of chemical gloves that have been contaminated with a toxic chemical is not advisable. For this reason, the disposal of gloves on a regular and frequent basis is advisable.
SUMMARYFarmers and pesticide applicators need to exercise extreme care in the selection, care, and reuse of chemical-resistant gloves. Understanding selection criteria, glove limitations, and proper care, and adhering to safe handling procedures can eliminate most accidental exposures.
Don't ever believe that a glove can solve all problems when handling toxic chemicals. The fact is no "impermeable" plastic or rubber material exists and no one material serves as a barrier to all chemicals.
YOU are the key to hand safety and protection from chemicals. You must determine suitability based on your own performance requirements. Remember-your safety and health are in your hands.
DEFINITIONS- Breakthrough Time - The time which elapses between initial contact of a chemical with the outside surface of a protective material and when the chemical can be detected at the surface of the material.
- Co-polymer - A long chain molecule synthesized by reaction of more than one monomer species with each other. Copolymers often have cost and/or performance advantages over polymers.
- Degradation - A reduction in one of physical properties of a glove or protective clothing.
- Penetration - The movement of chemicals through zippers, stitched seams or imperfections (e.g., pinholes) in a protective clothing material.
- Permeation - Process by which a chemical can pass a protective film without going through pores, or other visible openings (eg., What happens to air in an inflated balloon after several hours? -- same principle).
- Polymer - A substance formed by the union of small molecules of the same kind (monomers).


